11. Which is not obtained when metal carbides react with $${H_2}O?$$
A
$$A{l_4}{C_3} + {H_2}O \to CH \equiv CH$$
B
$$Ca{C_2} + {H_2}O \to CH \equiv CH$$
C
$$M{g_4}{C_3} + {H_2}O \to C{H_3}C \equiv CH$$
D
$$B{e_2}C + {H_2}O \to C{H_4}$$
Answer :
$$A{l_4}{C_3} + {H_2}O \to CH \equiv CH$$
12. Which of the following has lowest thermal stability?
A
$$L{i_2}C{O_3}$$
B
$$N{a_2}C{O_3}$$
C
$${K_2}C{O_3}$$
D
$$R{b_2}C{O_3}$$
Answer :
$$L{i_2}C{O_3}$$
13.
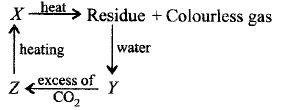
Identify $$X, Y$$ and $$Z.$$
| $$X$$ | $$Y$$ | $$Z$$ | |
| (a) | $$Ca{\left( {HC{O_3}} \right)_2}$$ | $$CaC{O_3}$$ | $$Ca{\left( {OH} \right)_2}$$ |
| (b) | $$CaC{O_3}$$ | $$Ca{\left( {OH} \right)_2}$$ | $$Ca{\left( {HC{O_3}} \right)_2}$$ |
| (c) | $$CaC{O_3}$$ | $$CaO$$ | $$Ca{\left( {OH} \right)_2}$$ |
| (d) | $$CaC{O_3}$$ | $$CaO$$ | $$Ca{\left( {HC{O_3}} \right)_2}$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
14. Both lithium and magnesium display several similar properties due to the diagonal relationship; however, the one which is incorrect is :
A
Both form basic carbonates
B
Both form soluble bicarbonates
C
Both form nitrides
D
Nitrates of both $$Li$$ and $$Mg$$ yield $$N{O_2}$$ and $${O_2}$$ on heating
Answer :
Both form basic carbonates
15. Which of the following statements is not correct regarding preparation of $$NaOH?$$
A
$$NaOH$$ is prepared by electrolysis of sodium chloride in Castner - Kellner cell.
B
Sodium metal discharged at cathode combines with mercury to form sodium amalgam.
C
Chlorine is evolved at anode.
D
Amalgam is heated to separate $$Na$$ and $$Hg.$$
Answer :
Amalgam is heated to separate $$Na$$ and $$Hg.$$
16. Which of the following metal ions play an important role in muscle contraction ?
A
$${K^ + }$$
B
$$N{a^ + }$$
C
$$M{g^{2 + }}$$
D
$$C{a^{2 + }}$$
Answer :
$$C{a^{2 + }}$$
17. A compound $$(A)$$ is used in preparation of washing soda to recover ammonia in Solvay's process. When $$C{O_2}$$ is bubbled through an aqueous solution of $$(A),$$ the solution turns milky. It is used in white washing due to disinfectant nature. What is the chemical formula of $$A?$$
A
$$Ca{\left( {HC{O_3}} \right)_2}$$
B
$$CaO$$
C
$$Ca{\left( {OH} \right)_2}$$
D
$$CaC{O_3}$$
Answer :
$$Ca{\left( {OH} \right)_2}$$
18. The sequence of ionic mobility in aqueous solution is
A
$${K^ + } > N{a^ + } > R{b^ + } > C{s^ + }$$
B
$$C{s^ + } > R{b^ + } > {K^ + } > N{a^ + }$$
C
$$R{b^ + } > {K^ + } > C{s^ + } > N{a^ + }$$
D
$$N{a^ + } > {K^ + } > R{b^ + } > C{s^ + }$$
Answer :
$$C{s^ + } > R{b^ + } > {K^ + } > N{a^ + }$$
19. Alkali metals are not found in free state due to their highly reactive nature. This is due to
A
their large size and low ionisation enthalpy
B
their large size and high ionisation enthalpy
C
their low ionisation enthalpy and high electron gain enthalpy
D
their tendency to impart colour to the flame
Answer :
their large size and low ionisation enthalpy
20. A metal $$M$$ readily forms its sulphate $$MS{O_4}$$ which is water soluble. It forms its oxide $$MO$$ which becomes inert on heating. It forms its insoluble hydroxide $$M{\left( {OH} \right)_2}$$ which is soluble in $$NaOH$$ solution. What would be $$M?$$
A
$$Be$$
B
$$Ba$$
C
$$Ca$$
D
$$Mg$$
Answer :
$$Be$$