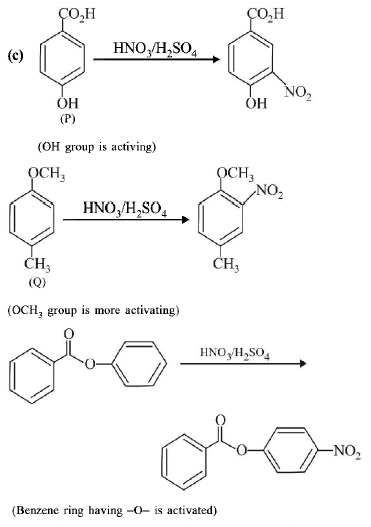
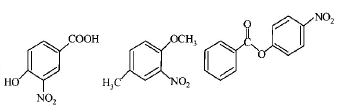
91.
The compounds $$P,$$ $$Q$$ and $$S$$
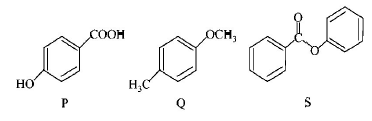
were separately subjected to nitration using $$HN{O_3}/{H_2}S{O_4}$$
mixture. The major product formed in each case respectively,
is :
A


B


C

D


Answer :


92. Carboxylic acids are more acidic than phenol and alcohol because of
A
intermolecular hydrogen bonding
B
formation of dimers
C
highly acidic hydrogen
D
resonance stabilization of their conjugate base
Answer :
resonance stabilization of their conjugate base
93. In the presence of a small amount of phosphorous, aliphatic carboxylic acids react with chlorine or bromine to yield a compound in which \[\alpha \] - hydrogen has been replaced by halogen. This reaction is known as :
A
Wolff - Kishner reaction
B
Rosenmund reaction
C
Etard reaction
D
Hell - Volhard - Zelinsky reaction
Answer :
Hell - Volhard - Zelinsky reaction
94. In the mechanism of Hoffmann reaction, which intermediate rearranges to alkyl isocyanate ?
A
Bromamide
B
Nitrene
C
Nitroso
D
Amide
Answer :
Nitrene
95. $$\alpha $$ - Hydroxypropanoic acid can be prepared from ethanal by following the steps given in the sequence.
A
Treat with $$HCN$$ followed by acidic hydrolysis.
B
Treat with $$NaHS{O_3}$$ followed by reaction with $$N{a_2}C{O_3}.$$
C
Treat with $${H_2}S{O_4}$$ followed by hydrolysis.
D
Treat with $${K_2}C{r_2}{O_7}$$ in presence of sulphuric acid.
Answer :
Treat with $$HCN$$ followed by acidic hydrolysis.
96. Benzoic acid may be converted into ethyl benzoate by reaction with
A
sodium ethoxide
B
ethyl chloride
C
dry $$HCl,{C_2}{H_5}OH$$
D
ethanol
Answer :
dry $$HCl,{C_2}{H_5}OH$$
97.
Identify $$(X)$$ and $$(Y)$$ in the given reaction sequence.
 \[\xrightarrow[KOH]{KMn{{O}_{4}}}X\xrightarrow{{{H}_{3}}{{O}^{+}}}Y\]
\[\xrightarrow[KOH]{KMn{{O}_{4}}}X\xrightarrow{{{H}_{3}}{{O}^{+}}}Y\]
A


B


C


D


Answer :


98. Phenol on treatment with $$C{O_2}$$ in the presence of $$NaOH$$ followed by acidification produces compound $$X$$ as the major product. $$X$$ on treatment with $${\left( {C{H_3}CO} \right)_2}O$$ in the presence of catalytic amount of $${H_2}S{O_4}$$ produces :
A
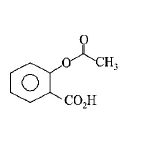

B
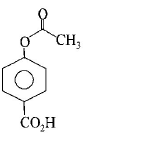

C
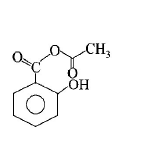

D
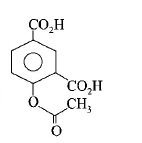

Answer :


99.
Identify the correct order of boiling points of the following compounds :
\[\underset{1}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH}}\,\,\,\,\underset{2}{\mathop{\,C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO}}\,\] \[\underset{3}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH}}\,\]
A
1 > 2 > 3
B
3 > 1 > 2
C
1 > 3 > 2
D
3 > 2 > 1
Answer :
3 > 1 > 2
100.
The correct order of strengths of the carboxylic acids

A
I > II > III
B
II > III > I
C
III > II > I
D
II > I > III
Answer :
II > III > I