101. When $$C{H_2} = CH - COOH$$ is reduced with $$LiAl{H_4},$$ the compound obtained will be
A
$$C{H_2} = CH - C{H_2}OH$$
B
$$C{H_3} - C{H_2} - C{H_2}OH$$
C
$$C{H_3} - C{H_2} - CHO$$
D
$$C{H_3} - C{H_2} - COOH$$
Answer :
$$C{H_2} = CH - C{H_2}OH$$
102.
A compound $$(X)$$ having molecular formula $${C_4}{H_8}{O_2}$$ is hydrolysed by water in presence of an acid to give a carboxylic acid $$(Y)$$ and an alcohol $$(Z).$$ $$(Z)$$ on oxidation with chromic acid gives $$(Y).$$ $$(X), (Y)$$ and $$(Z)$$ are
| $$X$$ | $$Y$$ | $$Z$$ | |
|---|---|---|---|
| (a) | $$C{H_3}COOC{H_3}$$ | $$C{H_3}COOH$$ | $$C{H_3}OH$$ |
| (b) | $$C{H_3}COO{C_2}{H_5}$$ | $$C{H_3}COOH$$ | $${C_2}{H_5}OH$$ |
| (c) | $${C_2}{H_5}COOC{H_3}$$ | $${C_2}{H_5}COOH$$ | $${C_2}{H_5}OH$$ |
| (d) | $$C{H_3}COO{C_2}{H_5}$$ | $${C_2}{H_5}COOH$$ | $$C{H_3}OH$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
103.
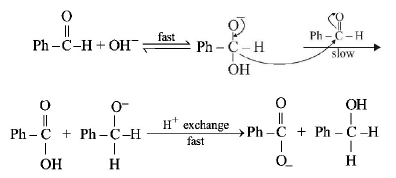
In Cannizzaro reaction given below
\[2PhCHO\xrightarrow{:\,\overset{\Theta }{\mathop{O}}\,H}PhC{{H}_{2}}OH+PhC\overset{\centerdot \centerdot }{\mathop{O}}\,_{2}^{\Theta }\]
the slowest step is :
A
the transfer of hydride to the carbonyl group
B
the abstraction of proton from the carboxylic group
C
the deprotonation of \[Ph\,C{{H}_{2}}OH\]
D
the attack of : \[\overset{\Theta }{\mathop{OH}}\,\] at the carboxyl group
Answer :
the transfer of hydride to the carbonyl group
104.
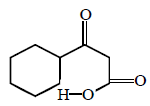 \[\xrightarrow{\Delta }\left( A \right)\xrightarrow{Zn\left( Hg \right)/HCl}\left( B \right)\] In the above reaction, product $$(B)$$ is :
\[\xrightarrow{\Delta }\left( A \right)\xrightarrow{Zn\left( Hg \right)/HCl}\left( B \right)\] In the above reaction, product $$(B)$$ is :
A
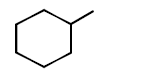

B
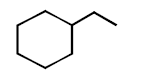

C
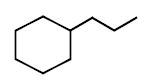

D
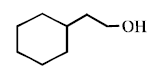

Answer :


105.
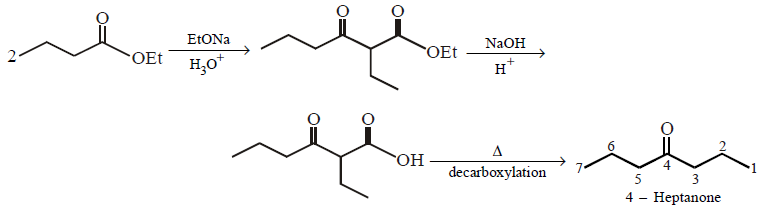
The correct product of the following sequence of reactions 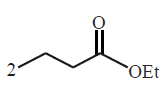 \[\xrightarrow[{{H}_{3}}{{O}^{+}}]{EtONa}\,\,\xrightarrow[{{H}_{3}}{{O}^{+}}]{NaOH,\,{{H}_{2}}O,\,\Delta }\,\,\xrightarrow{\Delta }?\]
\[\xrightarrow[{{H}_{3}}{{O}^{+}}]{EtONa}\,\,\xrightarrow[{{H}_{3}}{{O}^{+}}]{NaOH,\,{{H}_{2}}O,\,\Delta }\,\,\xrightarrow{\Delta }?\]
A
4 - heptanone
B
4 - methyl - 3 - hexanone
C
2 - ethyl pentanoic acid
D
2 - propyl butanoic acid
Answer :
4 - heptanone
106.
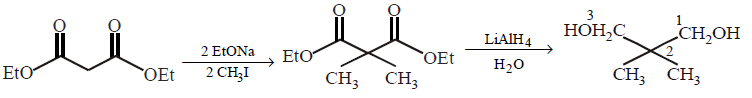
The correct product of the following reactions 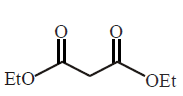 \[\xrightarrow[2\,C{{H}_{3}}I]{2\,EtONa}\,\,\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O]{\left( \text{i} \right)\,LiAl{{H}_{4}}}\]
\[\xrightarrow[2\,C{{H}_{3}}I]{2\,EtONa}\,\,\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O]{\left( \text{i} \right)\,LiAl{{H}_{4}}}\]
A
2, 2 - dimethyl propane diol
B
2 - methyl - 1 - propanol
C
2, 2 - dimethyl propanedioic acid
D
2 - methyl propanoic acid
Answer :
2, 2 - dimethyl propane diol
107.
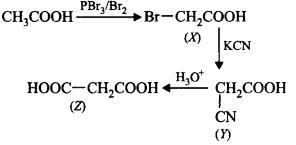
Complete the missing links $$(X), (Y)$$ and $$(Z)$$ by making an appropriate choice.
\[C{{H}_{3}}COOH\xrightarrow{\frac{PB{{r}_{3}}}{B{{r}_{2}}}}X\xrightarrow{KCN}\] \[Y\xrightarrow{{{H}_{3}}{{O}^{+}}}Z\]
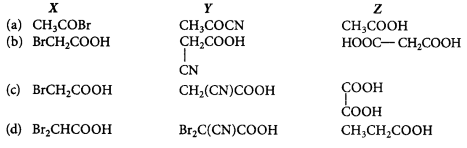
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
108. Which of the following is the correct order of relative strength of acids?
A
$$ClC{H_2}COOH > BrC{H_2}COOH$$ $$ > FC{H_2}COOH$$
B
$$BrC{H_2}COOH > ClC{H_2}COOH$$ $$ > FC{H_2}COOH$$
C
$$FC{H_2}COOH > ClC{H_2}COOH$$ $$ > BrC{H_2}COOH$$
D
$$ClC{H_2}COOH > FC{H_2}COOH$$ $$ > BrC{H_2}COOH$$
Answer :
$$FC{H_2}COOH > ClC{H_2}COOH$$ $$ > BrC{H_2}COOH$$
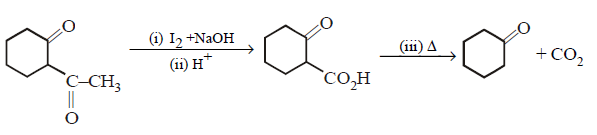
109.
End product of the following sequence of reactions are :
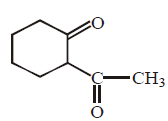 \[\xrightarrow[\begin{smallmatrix}
2.\,{{H}^{+}} \\
3.\,\Delta
\end{smallmatrix}]{1.\,{{I}_{2}}+NaOH,\,\Delta }\]
\[\xrightarrow[\begin{smallmatrix}
2.\,{{H}^{+}} \\
3.\,\Delta
\end{smallmatrix}]{1.\,{{I}_{2}}+NaOH,\,\Delta }\]
A
yellow $$ppt.$$ of \[CH{{I}_{3}},\] 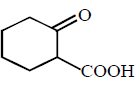

B
yellow $$ppt.$$ of \[CH{{I}_{3}},\]
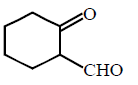

C
yellow $$ppt.$$ of \[CH{{I}_{3}},\] 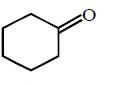

D
yellow $$ppt.$$ of \[CH{{I}_{3}},\] 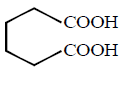

Answer :
yellow $$ppt.$$ of \[CH{{I}_{3}},\] 

110.
The major product of following reaction is :
\[R-C\equiv N\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O]{\left( \text{i} \right)\,AlH{{\left( \text{i}-Bu \right)}_{2}}}\]
A
$$RCOOH$$
B
$$RCON{H_2}$$
C
$$RCHO$$
D
$$RC{H_2}N{H_2}$$
Answer :
$$RCHO$$
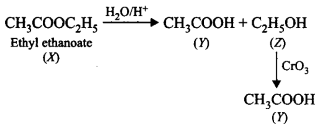
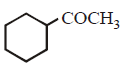 \[\xrightarrow[\text{Reduction}]{\text{Clemmensen}}\]
\[\xrightarrow[\text{Reduction}]{\text{Clemmensen}}\]