111.
In a set of the given reactions, acetic acid yielded a product $$C.$$
$$C{H_3}COOH + PC{l_5} \to $$ \[A\xrightarrow[anhy.\ AlC{{l}_{3}}]{{{C}_{6}}{{H}_{6}}}B\xrightarrow[\text{Ether}]{{{C}_{2}}{{H}_{5}}MgBr}C\]
Product $$C$$ would be
A
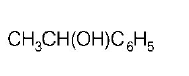
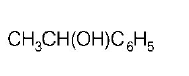
B


C


D
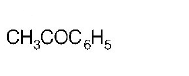
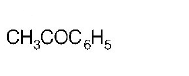
Answer :


112. An ester is boiled with $$KOH.$$ The product is cooled and acidified with concentrated $$HCl.$$ $$A$$ white crystalline acid separates. The ester is
A
methyl acetate
B
ethyl acetate
C
ethyl formate
D
ethyl benzoate
Answer :
ethyl benzoate
113. Which of the following statements is correct regarding formic acid?
A
It is a reducing agent.
B
It is a weaker acid than acetic acid.
C
It is an oxidising agent.
D
When its calcium salt is heated, it forms acetone.
Answer :
It is a reducing agent.
114. Which of the following represents the correct order of acidity in the given compounds?
A
$$FC{H_2}COOH > C{H_3}COOH$$ $$ > BrC{H_2}COOH > ClC{H_2}COOH$$
B
$$BrC{H_2}COOH > ClC{H_2}COOH$$ $$ > FC{H_2}COOH > C{H_3}COOH$$
C
$$FC{H_2}COOH > ClC{H_2}COOH$$ $$ > BrC{H_2}COOH > C{H_3}COOH$$
D
$$C{H_3}COOH > BrC{H_2}COOH$$ $$ > ClC{H_2}COOH < FC{H_2}COOH$$
Answer :
$$FC{H_2}COOH > ClC{H_2}COOH$$ $$ > BrC{H_2}COOH > C{H_3}COOH$$
115.
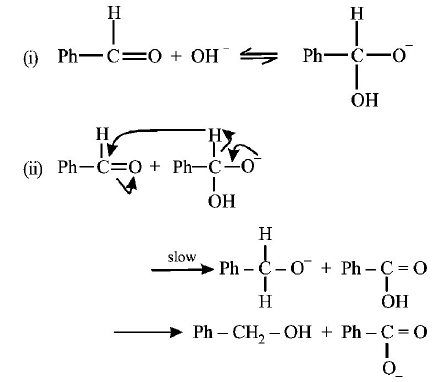
In the Cannizzaro reaction given below,
\[2PhCHO\xrightarrow{^{-}OH}PhC{{H}_{2}}OH+PhCO_{2}^{-},\]
the slowest step is
A
the attack of $$^ - OH$$ at the carbonyl group,
B
the transfer of hydride to the carbonyl group,
C
the abstraction of proton from the carboxylic acid,
D
the deprotonation of $$PhC{H_2}OH.$$
Answer :
the transfer of hydride to the carbonyl group,
116. A liquid was mixed with ethanol and a drop of concentrated $${H_2}S{O_4}$$ was added. A compound with a fruity smell was formed. The liquid was :
A
$$HCHO$$
B
$$C{H_3}COC{H_3}$$
C
$$C{H_3}COOH$$
D
$$C{H_3}OH$$
Answer :
$$C{H_3}COOH$$
117. Schotten-Baumann reaction is a reaction of phenols with
A
benzoyl chloride and $$NaOH$$
B
acetyl chloride and $$NaOH$$
C
salicylic acid and $$conc.\,{H_2}S{O_4}$$
D
acetyl chloride and $$conc.\,{H_2}S{O_4}$$
Answer :
benzoyl chloride and $$NaOH$$
118. $$ - OH$$ group present in alcohols is neutral while it is acidic in carboxylic acid because
A
in carboxylic acid $$ - OH$$ group is attached to electron withdrawing carbonyl group
B
in alcohols $$ - OH$$ group is attached to alkyl group which is electron withdrawing
C
carboxylic group is an electron releasing group
D
alcoholic group is an electron withdrawing group.
Answer :
in carboxylic acid $$ - OH$$ group is attached to electron withdrawing carbonyl group
119. When propionic acid is treated with aqueous sodium bicarbonate, $$C{O_2}$$ is liberated. The $$'C'$$ of $$C{O_2}$$ comes from
A
methyl group
B
carboxylic acid group
C
methylene group
D
bicarbonate
Answer :
bicarbonate
120. Which of the following acids has the smallest dissociation constant ?
A
\[C{{H}_{3}}CH\left( F \right)COOH\]
B
\[FC{{H}_{2}}C{{H}_{2}}COOH\]
C
\[BrC{{H}_{2}}C{{H}_{2}}COOH\]
D
\[C{{H}_{3}}CH\left( Br \right)COOH\]
Answer :
\[BrC{{H}_{2}}C{{H}_{2}}COOH\]