11. Acetic acid on heating in presence of \[{{P}_{2}}{{O}_{5}}\] gives
A
acetic anhydride
B
acetylene
C
peracid
D
no reaction
Answer :
acetic anhydride
12.
Picric acid is :
A


B


C
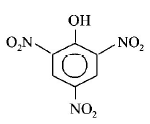

D
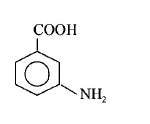

Answer :


13.
Fill in the blanks.
In Hell-Volhard-Zelinsky reaction, the carboxylic acids are halogenated at _______ position by using _______ and _______.
A
$$\alpha ,NaOH,{\text{iodine }}$$
B
$$\alpha ,{\text{phosphorus, halogen }}$$
C
$$\beta ,{\text{phosphorus, }}{H_2}O$$
D
$$\beta ,PC{l_5},NaOH$$
Answer :
$$\alpha ,{\text{phosphorus, halogen }}$$
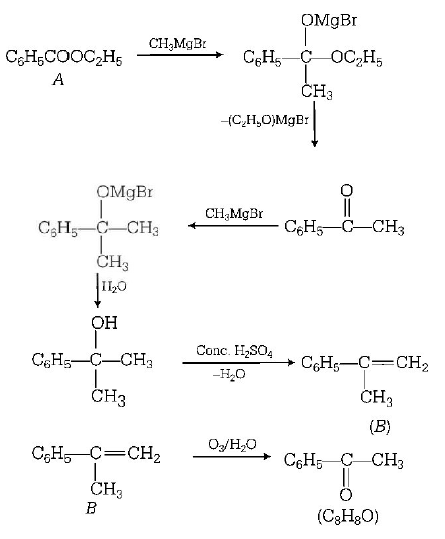
14. An ester $$(A)$$ with molecular formula $${C_9}{H_{10}}{O_2}$$ was treated with excess of $$C{H_3}MgBr$$ and the complex so formed was treated with $${H_2}S{O_4}$$ to give an olefin $$(B).$$ Ozonolysis of $$(B)$$ gave a ketone with molecular formula $${C_8}{H_8}O$$ which shows positive iodoform test. The structure of $$(A)$$ is
A
$${C_6}{H_5}COO{C_2}{H_5}$$
B
$${C_6}{H_5}COO{C_6}{H_5}$$
C
$${H_3}CCOO{C_6}{H_5}$$
D
$$p - {H_3}CO{C_6}{H_4}COC{H_3}$$
Answer :
$${C_6}{H_5}COO{C_2}{H_5}$$
15.
Identify $$(X), (Y)$$ and $$(Z)$$ in the given reaction.
\[C{{H}_{3}}CHO\xrightarrow[{{H}_{2}}S{{O}_{4}}]{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}\left( X \right)\xrightarrow{PC{{l}_{5}}}\] \[\left( Y \right)\xrightarrow[\text{Anhy}\text{.}\,AlC{{l}_{3}}]{{{C}_{6}}{{H}_{6}}}\left( Z \right)\]
A
$$\left( X \right) = HCOOH,\left( Y \right) = COC{l_2},$$ $$\left( Z \right) = {C_6}{H_5}Cl$$
B
$$\left( X \right) = C{H_3}COOH,$$ $$\left( Y \right) = C{H_3}COCl,$$ $$\left( Z \right) = {C_6}{H_5}COC{H_3}$$
C
$$\left( X \right) = C{H_3}COOH,$$ $$\left( Y \right) = C{H_3}C{H_2}C{H_2}Cl,$$ $$\left( Z \right) = {C_6}{H_5}C{H_2}C{H_2}C{H_3}$$
D
$$\left( X \right) = C{H_3}COC{H_3},$$ $$\left( Y \right) = C{H_3}CHClC{H_3},$$ $$\left( Z \right) = {C_6}{H_5}COC{H_3}$$
Answer :
$$\left( X \right) = C{H_3}COOH,$$ $$\left( Y \right) = C{H_3}COCl,$$ $$\left( Z \right) = {C_6}{H_5}COC{H_3}$$
16.
Identify the compounds $$(X), (Y)$$ and $$(Z)$$ in the following reaction :
\[C{{H}_{3}}Br\xrightarrow{\frac{Mg}{\text{ether}}}X\xrightarrow[\left( \text{ii} \right)\,\text{water}]{\left( \text{i} \right)\,C{{O}_{2}}}\] \[Y\xrightarrow[\Delta ]{C{{H}_{3}}OH,\,{{H}^{+}}}Z\]
A
$$\left( X \right) = C{H_3}MgBr,$$ $$\left( Y \right) = C{H_3}COOH,$$ $$\left( Z \right) = C{H_3}COOC{H_3}$$
B
$$\left( X \right) = C{H_3}C{H_2}Br,$$ $$\left( Y \right) = C{H_3}C{H_2}OH,$$ $$\left( Z \right) = C{H_3}C{H_2}C{H_2}C{H_3}$$
C
$$\left( X \right) = C{H_3}C{H_2}MgBr,$$ $$\left( Y \right) = C{H_3}C{H_2}COOH,$$ $$\left( Z \right) = C{H_3}C{H_2}COC{H_3}$$
D
$$\left( X \right) = C{H_3}COOH,$$ $$\left( Y \right) = C{H_3}C{H_2}COC{H_3},$$ $$\left( Z \right) = C{H_3}COOC{H_3}$$
Answer :
$$\left( X \right) = C{H_3}MgBr,$$ $$\left( Y \right) = C{H_3}COOH,$$ $$\left( Z \right) = C{H_3}COOC{H_3}$$
17. Phthalic acid reacts with resorcinol in the presence of concentrated \[{{H}_{2}}S{{O}_{4}}\] to give:
A
Phenolphthalein
B
Alizarin
C
Coumarin
D
Fluorescein
Answer :
Fluorescein
18.
Complete the reactions with appropriate products.
\[\left( \text{i} \right)C{{H}_{3}}CHO+N{{H}_{2}}OH\to X\]
\[\left( \text{ii} \right)C{{H}_{2}}=C{{H}_{2}}+PdC{{l}_{2}}+{{H}_{2}}O\] \[\xrightarrow{CuC{{l}_{2}}}Y\]
\[\left( \text{iii} \right)C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\] \[\xrightarrow{Cr{{O}_{3}},\,{{H}_{2}}S{{O}_{4}}}Z\]
A
$$X = C{H_3}CH = NOH,$$ $$Y = C{H_3}CHO,$$ $$Z = C{H_3}C{H_2}C{H_2}COOH$$
B
$$X = C{H_3}C{H_2}N{H_2},$$ $$Y = C{H_3}C{H_2}CHO,$$ $$Z = C{H_3}C{H_2}C{H_2}COOH$$
C
$$X = C{H_3}CON{H_2},Y = HCHO,$$ $$Z = C{H_3}COC{H_3}$$
D
$$X = C{H_3}C \equiv N,Y = C{H_3}CHO,Z = HCOOH$$
Answer :
$$X = C{H_3}CH = NOH,$$ $$Y = C{H_3}CHO,$$ $$Z = C{H_3}C{H_2}C{H_2}COOH$$
19. Identify $$Z$$ in the sequence \[C{{H}_{3}}COON{{H}_{4}}\xrightarrow{\Delta }X\xrightarrow[\Delta ]{{{P}_{2}}{{O}_{5}}}Y\xrightarrow{{{H}_{2}}O/{{H}^{+}}}Z\]
A
$$C{H_3}C{H_2}CON{H_2}$$
B
$$C{H_3}CN$$
C
$$C{H_3}COOH$$
D
$${\left( {C{H_3}CO} \right)_2}O$$
Answer :
$$C{H_3}COOH$$
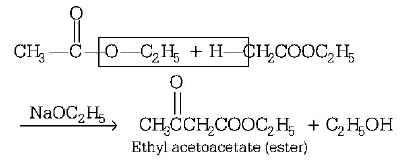
20. Self condensation of two moles of ethyl acetate in the presence of sodium ethoxide yields
A
ethyl butyrate
B
acetoacetic ester
C
methyl acetoacetate
D
ethyl propionate
Answer :
acetoacetic ester