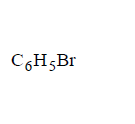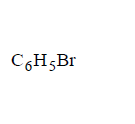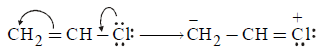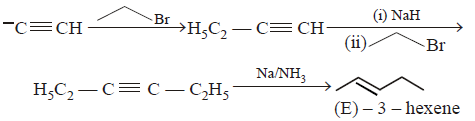81. Which chloride is least reactive with the hydrolysis point of view ?
A
$$C{H_3}Cl$$
B
$$C{H_3}C{H_2}Cl$$
C
$${\left( {C{H_3}} \right)_3}CCl$$
D
$$C{H_2} = CH - Cl$$
Answer :
$$C{H_2} = CH - Cl$$
82. The chief reaction product of reaction between $$n$$ - butane and bromine at $$573K$$ is :
A
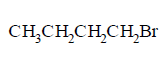
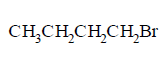
B
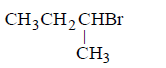
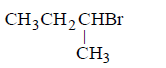
C
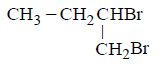
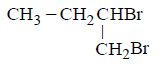
D
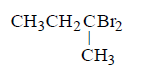
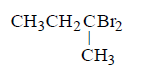
Answer :
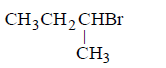
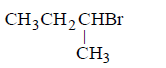
83. $${S_N}1$$ reaction is fastest in
A
\[C{{H}_{3}}C{{H}_{2}}Br\]
B
\[C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Cl\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\]
C
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,Cl\]
D
\[C{{H}_{3}}\underset{\begin{align}
& \,|\, \\
& C{{H}_{2}}\,\,\, \\
& \,| \\
& C{{H}_{3}}\,\,\, \\
\end{align}}{\mathop{-CH-}}\,Cl\]
Answer :
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,Cl\]
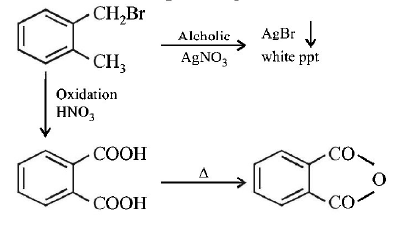
84. Compound (A), $${C_8}{H_9}Br,$$ gives a white precipitate when warmed with alcoholic $$AgN{O_3}$$ . Oxidation of (A) gives an acid (B), $${C_8}{H_6}{O_4}.$$ (B) easily forms anhydride on heating. Identify the compound (A).
A
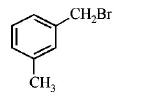

B
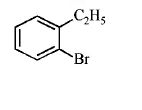

C
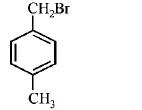

D
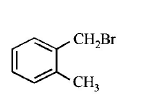

Answer :


85. Which one of the sequences below is the best synthesis of $$(E)-3-$$ hexene ?
A
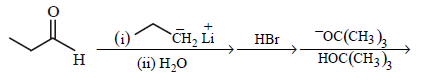
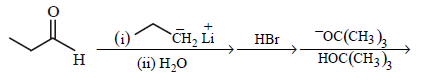
B
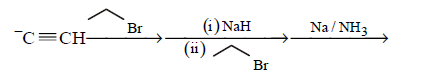
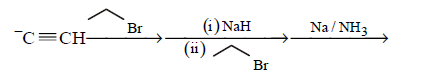
C
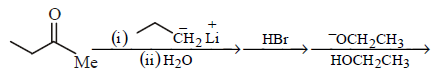
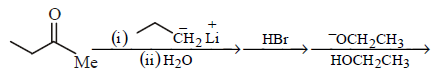
D
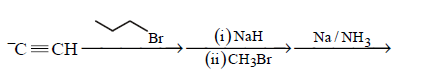
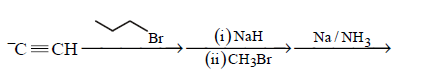
Answer :
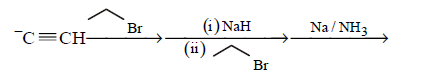
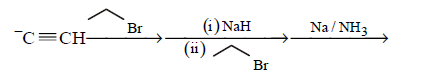
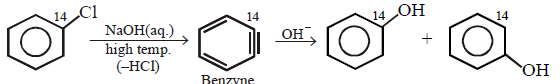
86.
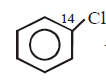 \[+NaOH\left( aq. \right)\xrightarrow{{{400}^{\circ }}C}\] product is
\[+NaOH\left( aq. \right)\xrightarrow{{{400}^{\circ }}C}\] product is
A
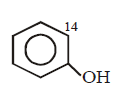

B
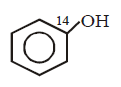

C


D
Answer :


87. Replacement of $$Cl$$ of chlorobenzene to give phenol requires drastic conditions but chlorine of 2, 4 - dinitrochlorobenzene is readily replaced. This is because
A
$$N{O_2}$$ makes the ring electron rich at ortho and para positions.
B
$$N{O_2}$$ withdraws $${e^ - }$$ from meta-position.
C
$$N{O_2}$$ donates $${e^ - }$$ at meta-position.
D
$$N{O_2}$$ withdraws $${e^ - }$$ from ortho/para-positions.
Answer :
$$N{O_2}$$ withdraws $${e^ - }$$ from ortho/para-positions.
88. Ethyl alcohol is obtained when ethyl chloride is boiled with
A
alcoholic $$KOH$$
B
aqueous $$KOH $$
C
water
D
aqueous $$KMn{O_4}$$
Answer :
aqueous $$KOH $$
89. Which one of the following chlorohydrocarbons readily undergoes solvolysis?
A
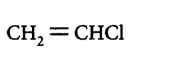
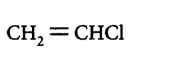
B
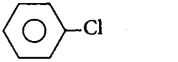
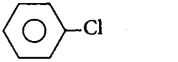
C
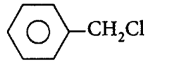

D
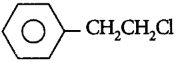
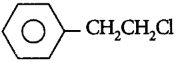
Answer :


90. Silver benzoate reacts with bromine to form
A
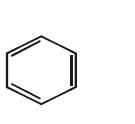

B
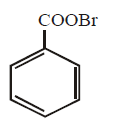

C
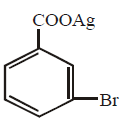

D
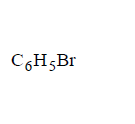
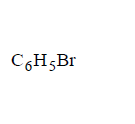
Answer :