161.
Among the following alkenes,
$$\mathop {1 - butene\,}\limits_{{\text{(i)}}} \,\,\,{\text{ }}\mathop {cis - 2 - butene}\limits_{{\text{(ii)}}} $$ $$\mathop {trans - 2 - butene}\limits_{{\text{(iii)}}} $$
the decreasing order of stability is
A
(ii) > (i) > (iii)
B
(iii) > (ii) > (i)
C
(iii) > (i) > (ii)
D
(i) > (ii) > (iii)
Answer :
(iii) > (ii) > (i)
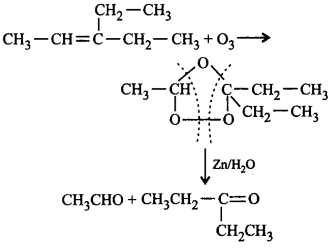
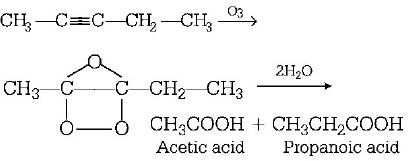
162. An alkene, 3-ethylpent-2-ene will give which of the following products on ozonolysis?
A
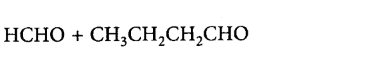
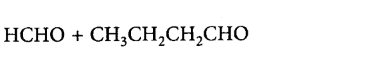
B


C
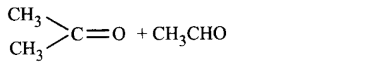
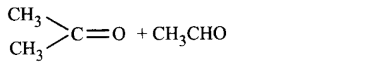
D


Answer :


163. Reduction of 2 - butyne with sodium in liquid ammonia gives predominantly
A
$$cis$$ - 2 - butene
B
$$trans$$ - 2 - butene
C
no reaction
D
$$n$$ - butane
Answer :
$$trans$$ - 2 - butene
164.
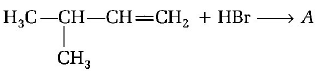
A (predominantly) is
A
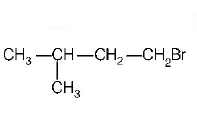

B


C


D


Answer :


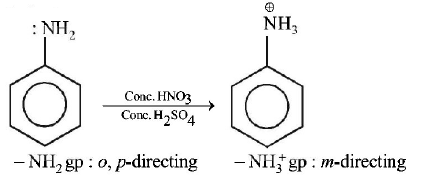
165. Which of the following compounds will form significant amount of meta product during mono-nitration reaction ?
A


B
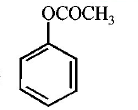

C
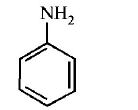

D


Answer :


166. How many monochlorobutanes will be obtained on chlorination of $$n$$ - butane ?
A
5
B
2
C
3
D
4
Answer :
2
167. Products of the following reaction\[C{{H}_{3}}C\equiv C\cdot C{{H}_{2}}C{{H}_{3}}\xrightarrow[\text{(ii)}\,\text{Hydrolysis}]{\text{(i)}\,{{O}_{3}}}\] ...are
A
$$C{H_3}CHO + C{H_3}C{H_2}CHO$$
B
$$C{H_3}COOH + C{H_3}COC{H_3}$$
C
$$C{H_3}COOH + HOOC \cdot C{H_2}C{H_3}$$
D
$$C{H_3}COOH + C{O_2}$$
Answer :
$$C{H_3}COOH + HOOC \cdot C{H_2}C{H_3}$$
168. Which one of the following compounds would have the highest heat of hydrogenation ?
A
$$C{H_2} = C{H_2}$$
B
$$C{H_3} - C{H_2} - CH = C{H_2}$$
C
$$C{H_3}CH = CHC{H_3}$$
D
$${\left( {C{H_3}} \right)_2}C = C{\left( {C{H_3}} \right)_2}$$
Answer :
$$C{H_2} = C{H_2}$$
169. Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
A
$$HCl > HBr > HI$$
B
$$HBr > HI > HCl$$
C
$$HI > HBr > HCl$$
D
$$HCl > HI > HBr$$
Answer :
$$HI > HBr > HCl$$
170. Which of the following reagents will be able to distinguish between 1-$$butyne$$ and 2-$$butyne?$$
A
$$NaN{H_2}$$
B
$$HCl$$
C
$${O_2}$$
D
$$B{r_2}$$
Answer :
$$NaN{H_2}$$