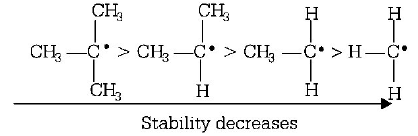
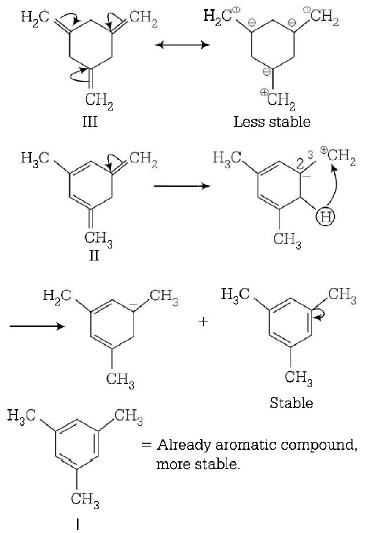
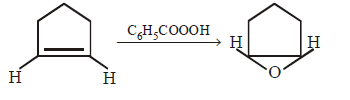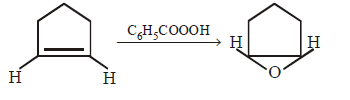181. Reactivity of hydrogen atoms attached to different carbon atoms in alkanes has the order
A
tertiary > primary > secondary
B
primary > secondary > tertiary
C
Both (A) and (B)
D
tertiary > secondary > primary
Answer :
tertiary > secondary > primary
182.
Fill in the blanks with appropriate words.
Benzene has a planar structure. All carbon atoms in benzene are $$\underline {\,\,{\text{I}}\,\,} $$ hybridised. The ring structure of benzene was proposed by $$\underline {\,\,{\text{II}}\,\,} .$$ It shows $$\underline {\,\,{\text{III}}\,\,} $$ substitution reactions. It reacts with $$\underline {\,\,{\text{IV}}\,\,} $$ in presence of aluminium chloride to form acetophenone.
| I | II | III | IV | |
|---|---|---|---|---|
| (a) | $$s{p^2}$$ | Kekule | electrophilic | acetyl chloride |
| (b) | $$sp$$ | Dewar | nucleophilic | chloromethane |
| (c) | $$s{p^3}$$ | Ladenberg | electrophilic | chloroethane |
| (d) | $$s{p^2}$$ | Baeyer | nucleophilic | methyl bromide |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(a)
183.
Given,

The enthalpy of hydrogenation of these compounds will be in the order as
A
I > II > III
B
III > II > I
C
II > III > I
D
II > I > III
Answer :
III > II > I
184. The highest boiling point is expected for :
A
iso-octane
B
$$n$$ - octane
C
2, 2, 3, 3 - tetramethylbutane
D
$$n$$ - butane
Answer :
$$n$$ - octane
185. 1-Bromo-3-chlorocyclobutane is treated with two equivalents of $$Na,$$ in the presence of ether. Which of the following compounds will be formed?
A


B
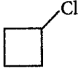

C
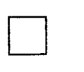

D
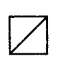

Answer :


186. The alkene $$R - CH = C{H_2}$$ reacts readily with $${B_2}{H_6}$$ and formed the product $$B$$ which on oxidation with alkaline $${H_2}{O_2}$$ produces
A
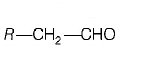
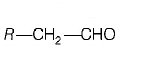
B
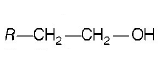
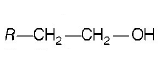
C
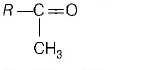
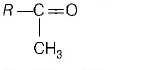
D
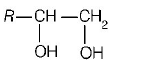

Answer :
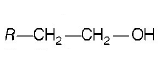
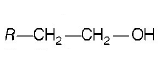
187.
Which step is chain propagation step in the following mechanism?
\[\begin{align}
& \left( \text{i} \right)C{{l}_{2}}\xrightarrow{h\upsilon }\dot{C}l+\dot{C}l \\
& \left( \text{ii} \right)\dot{C}l+C{{H}_{4}}\to \dot{C}{{H}_{3}}+HCl \\
& \left( \text{iii} \right)\dot{C}l+\dot{C}l\to C{{l}_{2}} \\
& \left( \text{iv} \right)\dot{C}{{H}_{3}}+\dot{C}l\to C{{H}_{3}}Cl \\
\end{align}\]
A
(i)
B
(ii)
C
(iii)
D
(iv)
Answer :
(ii)
188. \[R-C{{H}_{2}}-CC{{l}_{2}}-R\xrightarrow{\text{Reagent}}\] $$R - C \equiv C - R.$$ The reagent is
A
$$Na$$
B
$$HCl\,\,{\text{in}}\,\,{H_2}O$$
C
$$KOH\,\,{\text{in}}\,\,{C_2}{H_5}OH$$
D
$$Zn\,\,{\text{in alcohol}}$$
Answer :
$$KOH\,\,{\text{in}}\,\,{C_2}{H_5}OH$$
189.
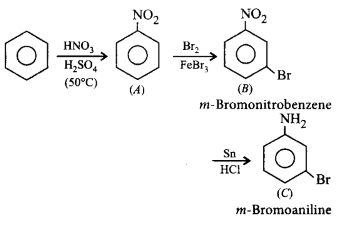
Identify $$(A), (B)$$ and $$(C)$$ in the following sequence of reactions.
 \[\xrightarrow[\begin{smallmatrix}
{{H}_{2}}S{{O}_{4}} \\
\left( {{50}^{\circ }}C \right)
\end{smallmatrix}]{HN{{O}_{3}}}\left( A \right)\xrightarrow[FeB{{r}_{3}}]{B{{r}_{2}}}\left( B \right)\] \[\xrightarrow[HCl]{Sn}\left( C \right)\]
\[\xrightarrow[\begin{smallmatrix}
{{H}_{2}}S{{O}_{4}} \\
\left( {{50}^{\circ }}C \right)
\end{smallmatrix}]{HN{{O}_{3}}}\left( A \right)\xrightarrow[FeB{{r}_{3}}]{B{{r}_{2}}}\left( B \right)\] \[\xrightarrow[HCl]{Sn}\left( C \right)\]
A
$$A \to $$ Nitrobenzene, $$B \to $$ Dinitrobenzene, $$C \to $$ $$p$$ - Bromoaniline
B
$$A \to {C_6}{H_5}S{O_3}H,B \to m$$ - Benzenesulphonic acid, $$C \to $$ $$m$$ - Benzenesulphonate
C
$$A \to {C_6}{H_5}N{O_2},B \to m$$ - Bromonitrobenzene, $$C \to $$ $$m$$ - Bromoaniline
D
$$A \to $$ $$p$$ - Nitrobenzene, $$B \to $$ $$m$$ - Trinitrobenzene, $$C \to $$ $$m$$ - Bromoaniline
Answer :
$$A \to {C_6}{H_5}N{O_2},B \to m$$ - Bromonitrobenzene, $$C \to $$ $$m$$ - Bromoaniline
190. Which of the following is correct ?
A
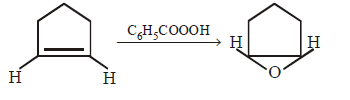
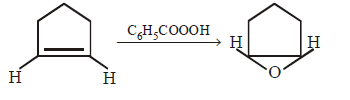
B
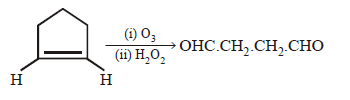
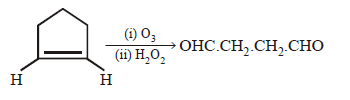
C
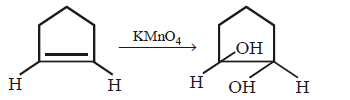

D
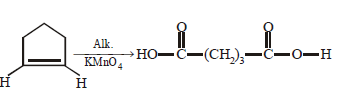

Answer :