91. The heats of combustion of carbon and carbon monoxide are $$-393.5$$ and $$ - 283.5\,kJ\,mo{l^{ - 1}}, $$ respectively. The heat of formation ( in $$kJ$$ ) of carbon monoxide per mole is :
A
-676.5
B
-110.5
C
110.5
D
676.5
Answer :
-110.5
92. One mole of an ideal gas at $$300\,K$$ is expanded isothermally from an initial volume of $$1\,litre$$ to $$10\,litre.$$ The $$\Delta E$$ for this process is $$\left( {R = 2\,cal\,mo{l^{ - 1}}{K^{ - 1}}} \right)$$
A
$$163.7\,cal$$
B
$$zero$$
C
$$1381.1\,cal$$
D
$$9\,lit\,atm$$
Answer :
$$zero$$
93. For the reaction, $${N_2} + 3{H_2} \rightleftharpoons 2N{H_3},\,\Delta H = ?$$
A
$$\Delta E + 2RT$$
B
$$\Delta E - 2RT$$
C
$$\Delta H = RT$$
D
$$\Delta E - RT$$
Answer :
$$\Delta E - 2RT$$
94. The entropy of a sample of a certain substance increases by $$0.836\,J\,{K^{ - 1}}$$ on adding reversibly $$0.3344\,J$$ of heat at constant temperature. The temperature of the sample is :
A
2.5$$\,K$$
B
0.3$$\,K$$
C
0.016$$\,K$$
D
0.4$$\,K$$
Answer :
0.4$$\,K$$
95. Which of the following pairs of a chemical reaction is certain to result in a spontaneous reaction?
A
Exothermic and decreasing disorder
B
Endothermic and increasing disorder
C
Exothermic and increasing disorder
D
Endothermic and decreasing disorder
Answer :
Exothermic and increasing disorder
96.
Consider the reactions given below. On the basis of these reactions find out which of the algebraic relations given in options (A) to (D) is correct ?
$$\left( {\text{i}} \right){C_{\left( g \right)}} + 4{H_{\left( g \right)}} \to C{H_{4\left( g \right)}};$$ $${\Delta _r}H = x\,kJ\,mo{l^{ - 1}}$$
$$\left( {{\text{ii}}} \right){C_{\left( {{\text{graphite}},s} \right)}} + 2{H_{2\left( g \right)}} \to C{H_{4\left( g \right)}};$$ $${\Delta _r}H = y\,kJ\,mo{l^{ - 1}}$$
A
$$x = y$$
B
$$x = 2y$$
C
$$x > y$$
D
$$x < y$$
Answer :
$$x > y$$
97.
Calculate the heat produced ( in $$kJ$$ ) when $$224\,g$$ of $$CaO$$ is completely converted to $$CaC{O_3}$$ by reaction with $$C{O_2}$$ at $${27^ \circ }C$$ in a container of fixed volume.
Given : $$\Delta H_f^ \circ \left( {CaC{O_3},s} \right) = - 1207\,kJ/mol;$$ $$\Delta H_f^ \circ \left( {CaO,s} \right) = - 635\,kJ/mol,$$ $$\Delta H_f^ \circ \left( {C{O_2}g} \right) = - 394\,kJ/mol;$$ $$\left[ {{\text{Use}}\,R = 8.3\,J{K^{ - 1}}\,mo{l^{ - 1}}} \right]$$
A
702.04$$\,kJ$$
B
721.96$$\,kJ$$
C
712$$\,kJ$$
D
721$$\,kJ$$
Answer :
702.04$$\,kJ$$
98. One mole of solid iron was vaporized in an oven at its boiling point of $$3443\,K$$ and enthalpy of vaporization of iron is $$344.3\,kJ\,mo{l^{ - 1}}.$$ The value of entropy vaporization $$\left( {{\text{in}}\,J\,mo{l^{ - 1}}} \right)$$ of iron is
A
100
B
10
C
-100
D
110
Answer :
100
99.
The free energy change for the following reactions are given below,
$${C_2}{H_2}\left( g \right) + \frac{5}{2}{O_2}\left( g \right) \to $$ $$2C{O_2}\left( g \right) + {H_2}O\left( l \right);$$ $$\Delta {G^ \circ } = - 1234kJ$$
$$C\left( s \right) + {O_2}\left( g \right) \to C{O_2}\left( g \right);$$ $$\Delta {G^ \circ } = - 394kJ$$
$${H_2}\left( g \right) + \frac{1}{2}{O_2}\left( g \right) \to {H_2}O\left( l \right);$$ $$\Delta {G^ \circ } = - 237kJ$$
What is the standard free energy change for the reaction $${H_2}\left( g \right) + 2C\left( s \right) \to {C_2}{H_2}\left( g \right)$$
A
- 209$$\,kJ$$
B
- 2259$$\,kJ$$
C
+ 2259$$\,kJ$$
D
209$$\,kJ$$
Answer :
209$$\,kJ$$
100.
A cyclic process $$ABCD$$ is shown in $$P–V$$ diagram for an ideal gas. Which of the following diagram represents the same process?
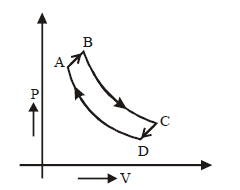
A
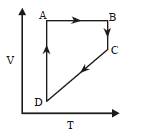

B
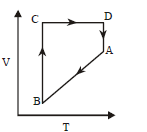

C


D
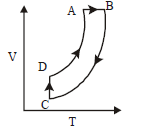

Answer :

