111.
A piston filled with $$0.04\,mol$$ of an ideal gas expands reversibly from $$50.0\,mL$$ to $$375\,mL$$ at a constant temperature of $${37.0^ \circ }C.$$ As it does so, it absorbs $$208\,J$$ of heat. The values of $$q$$ and $$w$$ for the process will be :
$$\left( {R = 8.314\,J/mol\,K} \right)\left( {ln\,7.5 = 2.01} \right)$$
A
$$q = + 208\,J,w = - 208\,J$$
B
$$q = - 208\,J,\,w = - 208\,J$$
C
$$q = - 208\,J,\,w = + 208\,J$$
D
$$q = + 208\,J,\,w = + 208\,J$$
Answer :
$$q = + 208\,J,w = - 208\,J$$
112. The enthalpies of combustion of carbon and carbon monoxide are $$- 393.5$$ and $$ - 283\,kJ\,mo{l^{ - 1}}$$ respectively. The enthalpy of formation of carbon monoxide per mole is
A
$$-676.5 kJ$$
B
$$676.5 kJ$$
C
$$110.5 kJ$$
D
$$-110.5 kJ$$
Answer :
$$-110.5 kJ$$
113. $$\Delta G$$ in $$A{g_2}O \to 2\,Ag + \frac{1}{2}{O_2}$$ at a certain temperature is $$ - 10\,kJ\,mo{l^{ - 1}}.$$ Pick the correct statement
A
$$A{g_2}O$$ decomposes to $$Ag$$ and $${O_2}$$
B
$$Ag$$ and $${O_2}$$ combines to form $$A{g_2}O$$
C
Reaction is in equilibrium
D
Reaction does not take place
Answer :
$$A{g_2}O$$ decomposes to $$Ag$$ and $${O_2}$$
114. $${\text{The}}\,\Delta H_f^0\,{\text{for}}\,C{O_2}\left( g \right),\,CO\left( g \right)\,$$ and $${H_2}O\left( g \right)$$ are $$-393.5,$$ $$-110.5$$ and $$ - 241.8\,kJ\,mo{l^{ - 1}}$$ respectively. The standard enthalpy change ( in $$kJ$$ ) for the reaction $$C{O_2}\left( g \right) + {H_2}\left( g \right) \to CO\left( g \right) + {H_2}O\left( g \right)\,{\text{is}}$$
A
524.1
B
41.2
C
-262.5
D
-41.2
Answer :
41.2
115.
Study the given graph and choose the correct option.
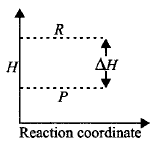
A
$$\Delta H = $$ Net heat absorbed from the surroundings
B
$$\Delta H = $$ Net heat given to the surroundings
C
$$\Delta H = + ve$$ for the reaction
D
$$\Delta H = $$ Total energy possessed by the reactants
Answer :
$$\Delta H = $$ Net heat given to the surroundings
116. Thermodynamics is not concerned about __________.
A
energy changes involved in a chemical reaction
B
the extent to which a chemical reaction proceeds
C
the rate at which a reaction proceeds
D
the feasibility of a chemical reaction
Answer :
the rate at which a reaction proceeds
117. The change in energy on freezing $$1.0\,kg$$ of liquid water at $${0^ \circ }C$$ and $$atm$$ is ( Given energy of 1 fusion of ice $$ = 6.03\,kJ\,mo{l^{ - 1}}$$ at $${0^ \circ }C$$ )
A
$$235\,kJ\,k{g^{ - 1}}$$
B
$$450\,kJ\,k{g^{ - 1}}$$
C
$$ - 335\,kJ\,k{g^{ - 1}}$$
D
$$ - 235\,kJ\,k{g^{ - 1}}$$
Answer :
$$ - 335\,kJ\,k{g^{ - 1}}$$
118. The heat of atomization of methane and ethane are $$360\,kJ/mol$$ and $$620\,kJ/mol,$$ respectively. The longest wavelength of light capable of breaking the $$C – C$$ bond is ( Avogadro number $$ = 6.02 \times {10^{23}}mo{l^{ - 1}},h = 6.62 \times {10^{ - 34}}J\,\left. s \right):$$
A
$$2.48 \times {10^4}\,nm$$
B
$$1.49 \times {10^3}\,nm$$
C
$$2.48 \times {10^3}\,nm$$
D
$$1.49 \times {10^4}\,nm$$
Answer :
$$1.49 \times {10^3}\,nm$$
119.
$$\left( {\Delta H - \Delta U} \right)$$ for the formation of carbon monoxide $$(CO)$$ from its elements at $$298 K$$ is
$$\left( {R = 8.314\,J{K^{ - 1}}\,mo{l^{ - 1}}} \right)$$
A
$$ - 2477.57\,J\,mo{l^{ - 1}}$$
B
$$2477.57\,J\,mo{l^{ - 1}}$$
C
$$ - 1238.78\,J\,mo{l^{ - 1}}$$
D
$$1238.78\,J\,mo{l^{ - 1}}$$
Answer :
$$1238.78\,J\,mo{l^{ - 1}}$$
120. For a reaction, $$P + Q \to R + S.$$ The value of $$\Delta {H^ \circ }$$ is $$ - 30\,kJ\,mo{l^{ - 1}}$$ and $$\Delta S$$ is $$ - 100\,J\,{K^{ - 1}}\,mo{l^{ - 1}}.$$ At what temperature the reaction will be at equilibrium ?
A
$$27{\,^ \circ }C$$
B
$$52{\,^ \circ }C$$
C
$$30{\,^ \circ }C$$
D
$$45{\,^ \circ }C$$
Answer :
$$27{\,^ \circ }C$$