241.
Read the following statements regarding spontaneity of a process and mark the appropriate choice.
(i) When enthalpy factor is absent then randomness factor decides spontaneity of a process.
(ii) When randomness factor is absent then enthalpy factor decides spontaneity of a process.
(iii) When both the factors take place simultaneously, the magnitude of both the factors decide spontaneity of a process.
A
Statements (i) and (ii) are correct and (iii) is incorrect.
B
Statement (iii) is correct, (i) and (ii) are incorrect.
C
Statements (i), (ii) and (iii) are correct.
D
Statements (i), (ii) and (iii) are incorrect.
Answer :
Statements (i), (ii) and (iii) are correct.
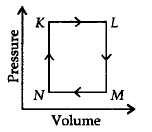
242.
Which thermochemical process is shown by the following figure ?
A
Standard enthalpy of a reaction
B
Born - Haber cycle of lattice enthalpy
C
Hess's law of constant heat summation
D
Standard enthalpy of a solution
Answer :
Hess's law of constant heat summation
243.
The molar entropies of $$HI\left( g \right)$$ and $$I\left( g \right)$$ at $$298\,K$$ are $$206.5,114.6,$$ and $$180.7\,J\,mo{l^{ - 1}}{K^{ - 1}}$$ respectively. Using the $$\Delta {G^ \circ }$$ given Below, calculate the bond energy of $$HI.$$
$$HI\left( g \right) \to H\left( g \right) + I\left( g \right);\Delta {G^ \circ } = 271.8\,kJ$$
A
$$282.4\,kJ\,mo{l^{ - 1}}$$
B
$$298.3\,kJ\,mo{l^{ - 1}}$$
C
$$290.1\,kJ\,mo{l^{ - 1}}$$
D
$$315.4\,kJ\,mo{l^{ - 1}}$$
Answer :
$$298.3\,kJ\,mo{l^{ - 1}}$$
244. For the process $${H_2}O\left( l \right)\left( {1\,{\text{bar}},\,373k} \right) \to {H_2}O\left( g \right)\left( {1\,{\text{bar}},\,373\,K} \right),$$ the correct set of thermodynamic parameters is
A
$$\Delta G = 0,\,\Delta S = + ve$$
B
$$\Delta G = 0,\Delta S = - ve$$
C
$$\Delta G = + ve,\Delta S = 0$$
D
$$\Delta G = - ve,\Delta S = + ve$$
Answer :
$$\Delta G = 0,\,\Delta S = + ve$$
245.
The enthalpy changes for the following processes are listed below :
$$\eqalign{
& C{l_2}\left( g \right) \to 2Cl\left( g \right),\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,242.3\,kJ\,mo{l^{ - 1}} \cr
& {I_2}\left( g \right) \to 2I\left( g \right),\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,151.0\,kJ\,mo{l^{ - 1}} \cr
& ICl\left( g \right) \to I\left( g \right) + Cl\left( g \right),\,\,\,\,211.3\,kJ\,mo{l^{ - 1}} \cr
& {I_2}\left( s \right) \to {I_2}\left( g \right),\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,62.76\,kJ\,mo{l^{ - 1}} \cr} $$
Given that the standard states for iodine and chlorine are $${I_2}\left( s \right)$$ and $$C{l_2}\left( g \right),$$ the standard enthalpy of formation for $$ICl\left( g \right)$$ is :
A
$$ + 16.8\,kJ\,mo{l^{ - 1}}$$
B
$$ + 244.8\,kJ\,mo{l^{ - 1}}$$
C
$$ - 14.6\,kJ\,mo{l^{ - 1}}$$
D
$$ - 16.8\,kJ\,mo{l^{ - 1}}$$
Answer :
$$ + 16.8\,kJ\,mo{l^{ - 1}}$$
246. The species which by definition has ZERO standard molar enthalpy of formation at $$298 K$$ is
A
$$B{r_2}\left( g \right)$$
B
$$C{l_2}\left( g \right)$$
C
$${H_2}O\left( g \right)$$
D
$$C{H_4}\left( g \right)$$
Answer :
$$C{l_2}\left( g \right)$$
247.
For the reaction : $${H_{2\left( g \right)}} + C{l_{2\left( g \right)}} \to 2HCl;\Delta H = - 44\,kcal$$
What is the enthalpy of decomposition of $$HCl?$$
A
$$ + 44\,kcal/mol$$
B
$$ - 44\,kcal/mol$$
C
$$ - 22\,kcal/mol$$
D
$$ + 22\,kcal/mol$$
Answer :
$$ + 22\,kcal/mol$$
248.
Consider the following reactions,
$$\left( {\text{i}} \right){H^ + }\left( {aq} \right) + O{H^ - }\left( {aq} \right) \to $$ $${H_2}O\left( l \right), - {x_1}\,kJ\,mo{l^{ - 1}}$$
$$\left( {{\text{ii}}} \right)\,{H_2}\left( g \right) + \frac{1}{2}{O_2}\left( g \right) \to $$ $${H_2}O\left( l \right),{x_2}\,kJ\,mo{l^{ - 1}}$$
$$\left( {{\text{iii}}} \right)C{O_2}\left( g \right) + {H_2}\left( g \right) \to $$ $$CO\left( g \right) + {H_2}O\left( l \right), - {x_3}\,kJ\,mo{l^{ - 1}}$$
$$\left( {{\text{iv}}} \right){C_2}{H_2}\left( g \right) + \frac{5}{2}{O_2}\left( g \right) \to $$ $$2\,C{O_2}\left( g \right) + {H_2}O\left( l \right), + {x_4}\,kJ\,mo{l^{ - 1}}$$
Enthalpy of formation of $${H_2}O\left( l \right)$$ is
A
$$ - {x_2}\,kJ\,mo{l^{ - 1}}$$
B
$$ + {x_3}\,kJ\,mo{l^{ - 1}}$$
C
$$ - {x_4}\,kJ\,mo{l^{ - 1}}$$
D
$$ + {x_1}\,kJ\,mo{l^{ - 1}}$$
Answer :
$$ - {x_2}\,kJ\,mo{l^{ - 1}}$$
249. The heat of combustion of ethane and benzene is $$ - 1560$$ and $$ - 3268\,kJ\,mo{l^{ - 1}}$$ respectively. Which of two has higher efficiencyasfuel per gram and what is the amount of heat produced per gram ?
A
$${\text{Benzene,}}41.9\,kJ\,{g^{ - 1}}$$
B
$${\text{Ethane,}}52\,kJ\,{g^{ - 1}}$$
C
$${\text{Benzene}},78\,kJ\,{g^{ - 1}}$$
D
$${\text{Ethane,}}30\,kJ\,{g^{ - 1}}$$
Answer :
$${\text{Ethane,}}52\,kJ\,{g^{ - 1}}$$
250.
A fixed mass $$'m'$$ of a gas is subjected to transformation
of states from $$K$$ to $$L$$ to $$M$$ to $$N$$ and back to $$K$$ as shown in the figure :
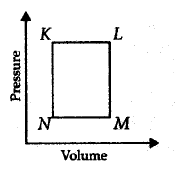
The succeeding operations that enable this transformation of states are
A
heating, cooling, heating, cooling
B
cooling, heating, cooling, heating
C
heating, cooling, cooling, heating
D
cooling, heating, heating, cooling
Answer :
heating, cooling, cooling, heating