301. The difference between heats of reaction at constant pressure and constant volume for the reaction : $$2{C_6}{H_6}\left( l \right) + 15{O_{2\left( g \right)}} \to $$ $$12C{O_2}\left( g \right) + 6{H_2}O\left( l \right)$$ at $${25^ \circ }C$$ in $$kJ$$ is
A
$$-$$ 7.43
B
$$+$$ 3.72
C
$$-$$ 3.72
D
$$+$$ 7.43
Answer :
$$-$$ 7.43
302. The enthalpy of neutralisation of a weak acid in $$1\,M$$ solution with a strong base is $$ - 56.1\,kcal\,mo{l^{ - 1}}.$$ If the enthalpy of ionisation of acid is $$1.5\,kcal\,mo{l^{ - 1}}$$ and enthalpy of neutralisation of the strong acid with a strong base is $$ - 57.3\,kJ\,e{q^{ - 1}}.$$ What is the $$\% $$ ionisation of the weak acid in molar solution ( assume the acid is monobasic )
A
25
B
20
C
15
D
10
Answer :
20
303. $$2\,moles$$ of an ideal gas at $${27^ \circ }C$$ temperature is expanded reversibly from $$2\,L$$ to $$20\,L.$$ Find entropy change $$\left( {R = 2\,cal/mol\,K} \right).$$
A
92.1
B
0
C
4
D
9.2
Answer :
9.2
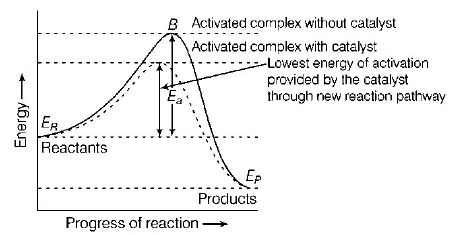
304. The addition of a catalyst during a chemical reaction alters which of the following quantities?
A
Internal energy
B
Enthalpy
C
Activation energy
D
Entropy
Answer :
Activation energy
305. The work done during the expansion of a gas from $$4\,d{m^3}$$ to $$6\,d{m^3}$$ against a constant external pressure of $$3\,atm$$ is $$\left( {1\,L\,atm = 101.32\,J} \right)$$
A
$$ - 6\,J$$
B
$$ - 608\,J$$
C
$$ + 304\,J$$
D
$$ - 304\,J$$
Answer :
$$ - 608\,J$$
306. Four grams of graphite is burnt in a bomb calorimeter of heat capacity $$30\,kJ\,{K^{ - 1}}$$ in excess of oxygen at 1 atmospheric pressure. The temperature rises from $$300$$ to $$304\,K$$ What is the enthalpy of combustion of graphite $$\left( {{\text{in}}\,\,kJ\,\,mo{l^{ - 1}}} \right)?$$
A
360
B
1440
C
- 360
D
- 1440
Answer :
- 360
307. What is the entropy change when $$1$$ $$mole$$ oxygen gas expands isothermally and reversibly from an initial volume of $$10\,L$$ to $$100\,L$$ at $$300\,K?$$
A
$$19.14\,J\,{K^{ - 1}}$$
B
$$109.12\,J\,{K^{ - 1}}$$
C
$$29.12\,J\,{K^{ - 1}}$$
D
$$10\,J\,{K^{ - 1}}$$
Answer :
$$19.14\,J\,{K^{ - 1}}$$
308. Temperature of $$5\,moles$$ of a gas is decreased by $$2K$$ at constant pressure. Indicate the correct statement
A
Work done by gas is $$ = 5R$$
B
Work done over the gas is $$ = 10\,R$$
C
Work done by the gas $$ = 10\,R$$
D
Work done $$ = 0$$
Answer :
Work done over the gas is $$ = 10\,R$$
309. Bond dissociation energies of $${H_2},C{l_2}$$ and $$HC{l_{\left( g \right)}}$$ are 104, 58 and 103$$\,kcal\,mo{l^{ - 1}}$$ respectively. Calculate the enthalpy of formation of $$HCl$$ gas
A
$$ - 22\,kcal$$
B
$$ + 22\,kcal$$
C
$$ + 184\,kcal$$
D
$$ - 184\,kcal$$
Answer :
$$ - 22\,kcal$$
310. During isothermal expansion of an ideal gas, its
A
internal energy increases
B
enthalpy decreases
C
enthalpy remains unaffected
D
enthalpy reduces to zero
Answer :
enthalpy remains unaffected