161. Which of the following statements is incorrect regarding the importance of coordination compounds in biological systems?
A
Vitamin $${B_{12}}$$ used to prevent anaemia is a complex compound of zinc.
B
Haemoglobin is the red pigment of blood and contains iron.
C
Chlorophyll is the green pigment of plants and contains magnesium.
D
All are correct
Answer :
Vitamin $${B_{12}}$$ used to prevent anaemia is a complex compound of zinc.
162. Which of the following is not correctly matched?
A
Coordination compound containing cationic complex ion : $${\left[ {Fe{{\left( {{H_2}O} \right)}_2}{{\left( {{C_2}{O_4}} \right)}_2}} \right]_2}S{O_4}$$
B
Coordination compound containing anionic complex ion : $$\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]Cl$$
C
Non-ionic coordination compound : $$\left[ {Co{{\left( {N{O_2}} \right)}_3}{{\left( {N{H_3}} \right)}_3}} \right]$$
D
Coordination compound containing cationic and anionic complex ion : $$\left[ {Pt{{\left( {N{H_3}} \right)}_4}} \right]\left[ {CuC{l_4}} \right]$$
Answer :
Coordination compound containing anionic complex ion : $$\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]Cl$$
163.
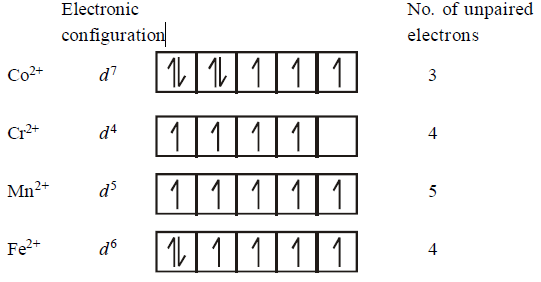
The $$d$$ - electron configurations of $$C{r^{2 + }},M{n^{2 + }},F{e^{2 + }}$$ and $$C{o^{2 + }}$$ are $${d^4},{d^5},{d^6}$$ and $${d^7}$$ respectively. Which one of the following will exhibit the lowest paramagnetic behaviour ?
$$\left( {{\text{Atomic no}}{\text{. }}Cr = 24,Mn = 25,Fe = 26,Co = 27} \right).$$
A
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
C
$${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
164. Which of the following will give maximum number of isomers ?
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]$$
B
$${\left[ {Ni\left( {en} \right){{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
C
$${\left[ {Ni\left( {{C_2}{O_4}} \right){{\left( {en} \right)}_2}} \right]^{2 - }}$$
D
$${\left[ {Cr{{\left( {SCN} \right)}_2}{{\left( {N{H_3}} \right)}_4}} \right]^ + }$$
Answer :
$${\left[ {Cr{{\left( {SCN} \right)}_2}{{\left( {N{H_3}} \right)}_4}} \right]^ + }$$
165. Incorrect match is :
A
$${K^ + }{\left[ {Pt\left( {{C_2}{H_4}} \right)C{l_3}} \right]^ - }:$$ Zeise’s salt
B
$${\left[ {Co{{\left( {CO} \right)}_4}} \right]^ - }:$$ Bond order of $$CO - CO$$ bond is greater than one
C
$${\left[ {V{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$ absorb visible light
D
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}:$$ Inner orbital low spin complex
Answer :
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}:$$ Inner orbital low spin complex
166. Which of the following will exhibit maximum ionic conductivity ?
A
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
C
$$\left[ {Cu{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]$$
D
$$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$
Answer :
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
167. The lowest value of paramagnetism is shown by
A
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
B
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
D
$${\left[ {Mn{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
Answer :
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
168. The formula of the complex diamminechlorido(ethylenediamine)nitroplatinum(IV)chloride is
A
$$\left[ {Pt{{\left( {N{H_3}} \right)}_2}Cl\left( {en} \right)N{O_2}} \right]C{l_2}$$
B
$$Pt\left[ {Pt{{\left( {N{H_3}} \right)}_2}\left( {en} \right)C{l_2}N{O_2}} \right]$$
C
$$Pt\left[ {{{\left( {N{H_3}} \right)}_2}\left( {en} \right)N{O_2}} \right]C{l_2}$$
D
$$Pt\left[ {{{\left( {N{H_3}} \right)}_2}\left( {en} \right)N{O_2}C{l_2}} \right]$$
Answer :
$$\left[ {Pt{{\left( {N{H_3}} \right)}_2}Cl\left( {en} \right)N{O_2}} \right]C{l_2}$$
169. IUPAC name of $$\left[ {Pt{{\left( {N{H_3}} \right)}_2}Cl\left( {N{O_2}} \right)} \right]$$ is
A
platinum diaminechloronitrite
B
chloronitrito-$$N$$-ammineplatinum(II)
C
diamminechloridonitrito-$$N$$-platinum(II)
D
diamminechloronitrito-$$N$$-platinate(II)
Answer :
diamminechloridonitrito-$$N$$-platinum(II)
170.
Match the column I with column II and mark the appropriate choice.
| Column I (complex) | Column II (Oxidation state of central atom) | ||
|---|---|---|---|
| a. | $${K_3}\left[ {Co{{\left( {{C_2}{O_4}} \right)}_2}C{l_2}} \right]$$ | 1. | 0 |
| b. | $${\left[ {Pt\left( {{C_2}{H_4}} \right)C{l_3}} \right]^ - }$$ | 2. | +1 |
| c. | $$\left[ {Fe{{\left( {{H_2}O} \right)}_5}NO} \right]S{O_4}$$ | 3. | +3 |
| d. | $$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$ | 4. | +2 |
A
a - 2, b -1 , c - 4, d - 3
B
a - 4, b - 2, c - 1, d - 3
C
a - 3, b - 4, c - 2, d - 1
D
a - 1, b - 2, c - 3, d - 4
Answer :
a - 3, b - 4, c - 2, d - 1