171. An octahedral complex with molecular composition $$M.5{\text{ }}N{H_3}.Cl.S{O_4}$$ has two isomers, $$A$$ and $$B.$$ The solution of $$A$$ gives a white precipitate with $$AgN{O_3}$$ solution and the solution of $$B$$ gives white precipitate with $$BaC{l_2}$$ solution. The type of isomerism exhibited by the complex is :
A
Linkage isomerism
B
Ionisation isomerism
C
Coordinate isomerism
D
Geometrical isomerism
Answer :
Ionisation isomerism
172. The correct order of ligands in the trans-directing series is
A
$$C{N^ - } > CH_3^ - > NO_2^ - > B{r^ - }$$
B
$$C{N^ - } > B{r^ - } > NO_2^ - > CH_3^ - $$
C
$$B{r^ - } > NO_2^ - > C{N^ - } > CH_3^ - $$
D
$$CH_3^ - > C{N^ - } > NO_2^ - > B{r^ - }$$
Answer :
$$C{N^ - } > CH_3^ - > NO_2^ - > B{r^ - }$$
173. In which of the following complex hybridization of central metal is not same as that of donor atom of ligand
A
$$\left[ {Ni{{\left( {P{F_3}} \right)}_4}} \right]$$
B
$$\left[ {Fe{{\left( {dmg} \right)}_2}} \right]$$
C
$${\left[ {Zn{{\left( {en} \right)}_2}} \right]^{2 + }}$$
D
$${\left[ {Ni{{\left( {PM{e_3}} \right)}_4}} \right]^{2 + }}$$
Answer :
$$\left[ {Fe{{\left( {dmg} \right)}_2}} \right]$$
174. In $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3},$$ the number of covalent bonds is
A
3
B
6
C
9
D
18
Answer :
18
175. Relative to the average energy in the spherical crystal field, the $${t_{2g}}$$ orbitals is tetrahedral field is
A
$${\text{Raised by}}\left( {\frac{2}{5}} \right){\Delta _t}$$
B
$${\text{Lowered by }}\left( {\frac{2}{5}} \right){\Delta _t}$$
C
$${\text{Raised by}}\,\left( {\frac{3}{5}} \right){\Delta _t}$$
D
$${\text{Lowered by}}\,\left( {\frac{1}{5}} \right){\Delta _t}$$
Answer :
$${\text{Raised by}}\left( {\frac{2}{5}} \right){\Delta _t}$$
176. The correct IUPAC name of the coordination compound $${K_3}\left[ {Fe{{\left( {CN} \right)}_5}NO} \right]$$ is
A
potassium pentacyanonitrosylferrate(II)
B
potassium pentacyanonitroferrate(III)
C
potassium nitritopentacyanoferrate(IV)
D
potassium nitritepentacyanoiron(II)
Answer :
potassium pentacyanonitrosylferrate(II)
177.
The $$d$$ electron configurations of $$C{r^{2 + }},M{n^{2 + }},F{e^{2 + }}$$ and $$N{i^{2 + }}$$ are $$3{d^4},3{d^5},3{d^6}$$ and $$3{d^8}$$ respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour ?
$$\left( {{\text{At}}{\text{. no}}{\text{. of}}\,\,Cr = 24,Mn = 25,} \right.$$ $$\left. {Fe = 26,Ni = 28} \right)$$
A
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {Ni{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
C
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Ni{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
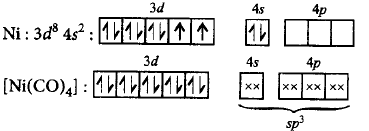
178. The geometry possessed by $$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$ is
A
tetrahedral
B
square planar
C
linear
D
octahedral
Answer :
tetrahedral
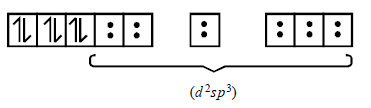
179. An octahedral complex of $$C{o^{3 + }}$$ is diamagnetic. The hybridisation involved in the formation of the complex is :
A
$$s{p^3}{d^2}$$
B
$$ds{p^2}$$
C
$${d^2}s{p^3}$$
D
$$s{p^3}d$$
Answer :
$${d^2}s{p^3}$$
180. A coordination compound $$X$$ gives pale yellow colour with $$AgN{O_3}$$ solution while its isomer $$Y$$ gives white precipitate with $$BaC{l_2}.$$ Two compounds are isomers of $$CoBrS{O_4} \cdot 5N{H_3}.$$ What could be the possible formula of $$X$$ and $$Y?$$
A
$$X = \left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Br,$$ $$Y = \left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]S{O_4}$$
B
$$X = \left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]S{O_4},$$ $$Y = \left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Br$$
C
$$X = \left[ {Co{{\left( {N{H_3}} \right)}_5}Br\left( {S{O_4}} \right)} \right],$$ $$Y = \left[ {CoBr\left( {S{O_4}} \right){{\left( {N{H_3}} \right)}_5}} \right]$$
D
$$X = \left[ {Co{{\left( {Br} \right)}_5}N{H_3}} \right]S{O_4},$$ $$Y = \left[ {CoBr\left( {S{O_4}} \right)} \right]N{H_3}$$
Answer :
$$X = \left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Br,$$ $$Y = \left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]S{O_4}$$