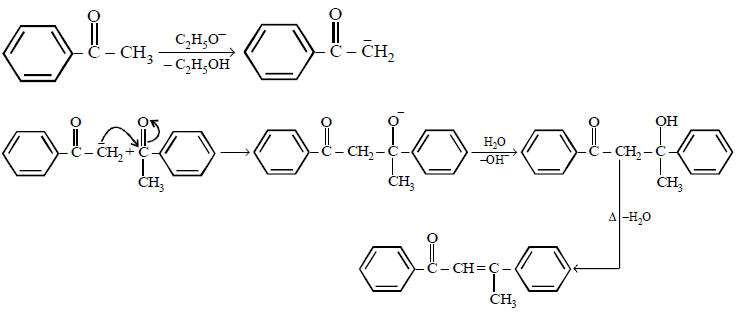
101. Acetophenone when reacted with a base, \[{{C}_{2}}{{H}_{5}}ONa,\] yields a stable compound which has the structure.
A


B


C


D


Answer :


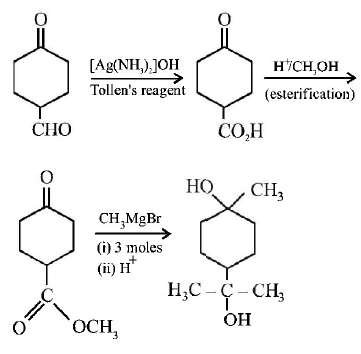
102.
The correct sequence of reagents for the following conversion will be :

A
$${\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }O{H^ - },{H^ + }/C{H_3}OH,C{H_3}MgBr$$
B
$$C{H_3}MgBr,{H^ + }/C{H_3}OH,{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }O{H^ - }$$
C
$$C{H_3}MgBr,{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }O{H^ - },{H^ + }/C{H_3}OH$$
D
$${\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }O{H^ - },C{H_3}MgBr,{H^ + }/C{H_3}OH$$
Answer :
$${\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }O{H^ - },{H^ + }/C{H_3}OH,C{H_3}MgBr$$
103. Benzaldehyde reacts with ethanolic $$KCN$$ to give
A
$${C_6}{H_5}CHOHCN$$
B
$${C_6}{H_5}CHOHCO{C_6}{H_5}$$
C
$${C_6}{H_5}CHOHCOOH$$
D
$${C_6}{H_5}CHOHCHOH{C_6}{H_5}$$
Answer :
$${C_6}{H_5}CHOHCO{C_6}{H_5}$$
104. The oxidation of toluene to benzaldehyde by chromyl chloride is called
A
Etard reaction
B
Riemer-Tiemann reaction
C
Wurtz reaction
D
Cannizzaro reaction
Answer :
Etard reaction
105. Which of the following will respond to Cannizzaro’s reaction ?
A
2, 2 - Dimethylpropanal
B
Acetaldehyde
C
Propionaldehyde
D
Cinnamaldehyde
Answer :
2, 2 - Dimethylpropanal
106.
In Cannizzaro reaction given below
\[2PhCHO\xrightarrow{:O{{H}^{-}}}PhC{{H}_{2}}OH+PhC\ddot{O}_{2}^{-}\]
the slowest step is :
A
the transfer of proton to the carbonyl group
B
the abstraction of proton from the carboxylic group
C
the deprotonation of \[Ph\,C{{H}_{2}}OH\]
D
the attack of : \[\bar{O}H\] at the carboxyl group
Answer :
the transfer of proton to the carbonyl group
107.
The correct structure of the product $$A$$ formed in the reaction
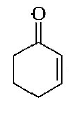 \[\xrightarrow[\text{Pd/carbon, ethanol}]{{{H}_{2}}\left( \text{gas, 1 atmosphere} \right)}A\] is
\[\xrightarrow[\text{Pd/carbon, ethanol}]{{{H}_{2}}\left( \text{gas, 1 atmosphere} \right)}A\] is
A


B
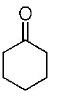

C
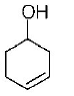

D
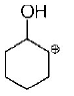

Answer :


108.
$$R - CH = CH - CHO + N{H_2}$$ \[\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,NHN{{H}_{2}}\xrightarrow{{{H}^{+}}}X\]
$$(X)$$ in the above reaction is
A
\[R-CH=CH\overset{\begin{smallmatrix}
\,OH \\
|\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,N{{H}_{2}}CONHN{{H}_{2}}\]
B
\[R-CH=CH-CH=\] \[N-NH\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,N{{H}_{2}}\]
C
\[R-CH=N{{H}_{2}}CON{{H}_{2}}\]
D
\[R-CH=CH\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,N{{H}_{2}}COCH\] \[=NHN{{H}_{2}}\]
Answer :
\[R-CH=CH-CH=\] \[N-NH\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,N{{H}_{2}}\]
109. Which of the following compounds does not react with $$NaHS{O_3}?$$
A
$$HCHO$$
B
$${C_6}{H_5}COC{H_3}$$
C
$$C{H_3}COC{H_3}$$
D
$$C{H_3}CHO$$
Answer :
$${C_6}{H_5}COC{H_3}$$
110. Which of the following is the most reactive isomer?
A
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,H\]
B
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{3}}\]
C
\[C{{H}_{3}}C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{2}}C{{H}_{3}}\]
D
\[C{{H}_{3}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\, \\
C{{H}_{3}}\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
Answer :
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,H\]