81.
The exothermic formation of $$CI{F_3}$$ is represented by the equation :
$$C{l_2}\left( g \right) + 3{F_2}\left( g \right) \rightleftharpoons 2CI{F_3}\left( g \right);\,\,\Delta H = - 329kJ$$
Which of the following will increase the quantity of $$Cl{F_3}$$ in an equilibrium mixture of $$C{l_2},$$ $${F_2}$$ and $$Cl{F_3}\,?$$
A
Adding $${F_2}$$
B
Increasing the volume of the container
C
Removing $$C{l_2}$$
D
Increasing the temperature
Answer :
Adding $${F_2}$$
82.
When $${I_2}$$ dissociates to its atomic form the following reaction occurs :
$${I_{2\left( g \right)}} \rightleftharpoons 2{I_{\left( g \right)}};\Delta {H^ \circ } = + 150\,kJ\,mo{l^{ - 1}}$$
The reaction is favoured at
A
low temperature
B
high temperature
C
no change with temperature
D
high pressure
Answer :
high temperature
83.
The equilibrium constant, $${K_c}$$ for the reaction of hydrogen with iodine is 57.0 at 700 $$K,$$ and the reaction is exothermic
The value of $${k_r}$$ at 700 $$K$$ is $$1.16 \times {10^{ - 3}}\,{M^{ - 1}}\,{s^{ - 1}}.$$ Which of the following is the correct value of $${k_f}?$$
A
$$1.26 \times {10^{ - 3}}\,{M^{ - 1}}\,{s^{ - 1}}$$
B
$$3.17 \times {10^{ - 2}}\,{M^{ - 1}}\,{s^{ - 1}}$$
C
$$6.61 \times {10^{ - 2}}\,{M^{ - 1}}\,{s^{ - 1}}$$
D
$$7.12 \times {10^{ - 3}}\,{M^{ - 1}}\,{s^{ - 1}}$$
Answer :
$$6.61 \times {10^{ - 2}}\,{M^{ - 1}}\,{s^{ - 1}}$$
84. $$3.2\,moles$$ of hydrogen iodide were heated in a sealed bulb at $${444^ \circ }C$$ till the equilibrium state was reached. Its degree of dissociation at this temperature was found to be $$22\% $$ The number of moles of hydrogen iodide present at equilibrium are
A
2.496
B
1.87
C
2
D
4
Answer :
2.496
85.
$$28g\,{N_2}$$ and $$6.0\,g$$ of $${H_2}$$ are heated over catalyst in a closed one litre flask of $${450^ \circ }C.$$ The entire equilibrium mixture required $$500\,mL$$ of $$1.0\,M\,{H_2}S{O_4}$$ for neutralisation. The value of $${K_c}$$ for the reaction
$${N_2}\left( g \right) + 3{H_2}\left( g \right) \rightleftharpoons 2\,N{H_3}\left( g \right)$$ is
A
$$0.06\,mo{l^{ - 2}}{L^2}$$
B
$$0.59\,mo{l^{ - 2}}{L^2}$$
C
$$1.69\,mo{l^2}{L^{ - 2}}$$
D
$$0.03\,mo{l^2}{L^{ - 2}}$$
Answer :
$$0.59\,mo{l^{ - 2}}{L^2}$$
86.
Consider the following graph and mark the correct statement.
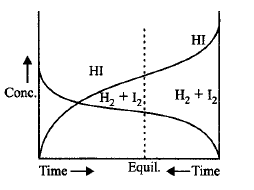
A
Chemical equilibrium in the reaction, $${H_2} + {I_2} \rightleftharpoons 2HI$$ can be attained from either directions.
B
Equilibrium can be obtained when $${H_2}$$ and $${I_2}$$ are mixed in an open vessel.
C
The concentrations of $${H_2}$$ and $${I_2}$$ keep decreasing while concentration of $$HI$$ keeps increasing with time.
D
We can find out equilibrium concentration of $${H_2}$$ and $${I_2}$$ from the given graph.
Answer :
Chemical equilibrium in the reaction, $${H_2} + {I_2} \rightleftharpoons 2HI$$ can be attained from either directions.
87. Change in volume of the system does not alter which of the following equilibria?
A
$${N_2}\left( g \right) + {O_2}\left( g \right) \rightleftharpoons 2NO\left( g \right)$$
B
$$PC{l_5}\left( g \right) \rightleftharpoons PC{l_3}\left( g \right) + C{l_2}\left( g \right)$$
C
$${N_2}\left( g \right) + 3{H_2}\left( g \right) \rightleftharpoons 2N{H_3}\left( g \right)$$
D
$$S{O_2}C{l_2}\left( g \right) \rightleftharpoons S{O_2}\left( g \right) + C{l_2}\left( g \right)$$
Answer :
$${N_2}\left( g \right) + {O_2}\left( g \right) \rightleftharpoons 2NO\left( g \right)$$
88.
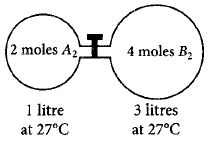
The gas $${A_2}$$ in the left flask allowed to react with gas $${B_2}$$ present in right flask as $${A_{2\left( g \right)}} + {B_{2\left( g \right)}} \rightleftharpoons 2A{B_{\left( g \right)}};{K_c} = 4$$ at $$27{\,^ \circ }C.$$ What is the concentration of $$AB$$ when equilibrium is established?
A
1.33 $$M$$
B
2.66 $$M$$
C
0.66 $$M$$
D
0.33 $$M$$
Answer :
0.66 $$M$$
89.
For the reaction,
$$C{H_4}\left( g \right) + 2\,{O_2}\left( g \right) \rightleftharpoons $$ $$C{O_2}\left( g \right) + 2\,{H_2}O\left( l \right),$$ $${\Delta _r}H = - 170.8\,kJ\,mo{l^{ - 1}}$$
Which of the following statement is not true ?
A
At equilibrium, the concentrations of $$C{O_2}\left( g \right)$$ and $${H_2}O\left( l \right)$$ are not equal
B
The equilibrium constant for the reaction is given by $${K_p} = \frac{{\left[ {C{O_2}} \right]}}{{\left[ {C{H_4}} \right]\left[ {{O_2}} \right]}}$$
C
Addition of $$C{H_4}\left( g \right)$$ or $${O_2}\left( g \right)$$ at equilibrium will cause a shift to the right
D
The reaction is exothermic
Answer :
The equilibrium constant for the reaction is given by $${K_p} = \frac{{\left[ {C{O_2}} \right]}}{{\left[ {C{H_4}} \right]\left[ {{O_2}} \right]}}$$
90. For the chemical reaction $$3X\left( g \right) + Y\left( g \right) \rightleftharpoons {X_3}Y\left( g \right),$$ the amount of $${X_3}Y$$ at equilibrium is affected by
A
temperature and pressure
B
temperature only
C
pressure only
D
temperature, pressure and catalyst
Answer :
temperature and pressure