301. The energies of activation for forward and reverse reactions for $${A_2} + {B_2} \rightleftharpoons 2AB$$ are $$180\,kJ\,mo{l^{ - 1}}$$ and $$200\,kJ\,mo{l^{ - 1}}$$ respectively, The presence of catalyst lowers the activation energy of both (forward and reverse) reactions by $$100\,kJ\,mo{l^{ - 1}}$$ . The enthalpy change of the reaction $$\left( {{A_2} + {B_2} \to 2AB} \right)$$ in the presence of a catalyst will be ( in $$kJ\,mo{l^{ - 1}}$$ )
A
20
B
300
C
120
D
280
Answer :
20
302. A first order reaction is $$50\% $$ completed in 20 minutes at $${27^ \circ }C$$ and in 5 minutes at $${47^ \circ }C.$$ The energy of activation of the reaction is :
A
$$43.85\,kJ/mol$$
B
$$55.14\,kJ/mol$$
C
$$11.97\,kJ/mol$$
D
$$6.65\,kJ/mol$$
Answer :
$$55.14\,kJ/mol$$
303.
Consider the Arrhenius equation given below and mark the correct option.
$$k = A{e^{ - \frac{{{E_a}}}{{RT}}}}$$
A
Rate constant increases exponentially with increasing activation energy and decreasing temperature.
B
Rate constant decreases exponentially with increasing activation energy and decreasing temperature.
C
Rate constant increases exponentially with decreasing activation energy and decreasing temperature.
D
Rate constant increases exponentially with decreasing activation energy and increasing temperature.
Answer :
Rate constant increases exponentially with decreasing activation energy and increasing temperature.
304.
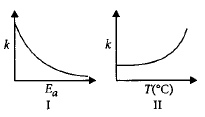
Consider the given plots for a reaction obeying Arrhenius equation $$\left( {{0^ \circ }C < T < 300{\,^ \circ }C} \right):$$ ( $$k$$ and $${E_a}$$ are rate constant and activation energy, respectively )
Choose the correct option.
A
I is right but II is wrong.
B
Both I and II are wrong.
C
I is wrong but II is right.
D
Both I and II are correct.
Answer :
Both I and II are correct.
305. Which of the following statements is incorrect?
A
Activation energy for the forward reaction is equals to activation energy for the reverse reaction
B
For a reversible reaction, an increase in temperature increases the reaction rate for
both the forward and the backward reaction
C
The larger the initial reactant concentration for a second order reaction, the shorter is
its half-life.
D
When $$\Delta t$$ is infinitesimally small, the average rate equals the instantaneous rate
Answer :
Activation energy for the forward reaction is equals to activation energy for the reverse reaction
306. The rate of reaction is doubled for every $${10^ \circ }C$$ rise in temperature. The increase in reaction rate as a result of temperature rise from $${10^ \circ }C$$ to $${100^ \circ }C$$ is
A
112
B
512
C
400
D
614
Answer :
512
307. Which of the following statements for order of reaction is not correct?
A
Order can be determined experimentally.
B
Order of reaction is equal to the sum of powers of concentration terms in rate law expression.
C
Order cannot be fractional.
D
Order is not affected by stoichiometric coefficient of the reactants.
Answer :
Order cannot be fractional.
308. The reaction $$X \to Y$$ is an exothermic reaction. Activation energy of the reaction for $$X$$ into $$Y$$ is $$150\,kJ\,mo{l^{ - 1}}.$$ Enthalpy of reaction is $$135\,kJ\,mo{l^{ - 1}}.$$ The activation energy for the reverse reaction, $$Y \to X$$ will be :
A
$$280\,kJ\,mo{l^{ - 1}}$$
B
$$285\,kJ\,mo{l^{ - 1}}$$
C
$$270\,kJ\,mo{l^{ - 1}}$$
D
$$15{\kern 1pt} \,kJ\,mo{l^{ - 1}}$$
Answer :
$$285\,kJ\,mo{l^{ - 1}}$$
309.
The following data were obtained during the first order thermal decomposition of $$S{O_2}C{l_2}$$ at a constant volume.
$$S{O_2}C{l_{2\left( g \right)}} \to S{O_{2\left( g \right)}} + C{l_{2\left( g \right)}}$$
| Experiment | Time/s-1 | Total pressure/atm |
|---|---|---|
| 1 | 0 | 0.5 |
| 2 | 100 | 0.6 |
What is the rate of reaction when total pressure is $$0.65\,atm?$$
A
$$0.35\,atm\,{s^{ - 1}}$$
B
$$2.235 \times {10^{ - 3}}\,atm\,{s^{ - 1}}$$
C
$$7.8 \times {10^{ - 4}}\,atm\,{s^{ - 1}}$$
D
$$1.55 \times {10^{ - 4}}\,atm\,{s^{ - 1}}$$
Answer :
$$7.8 \times {10^{ - 4}}\,atm\,{s^{ - 1}}$$
310. The rate constant for a first order reaction whose half life is $$480\,\sec ,$$ is :
A
$$1.44 \times {10^{ - 3}}{\sec ^{ - 1}}$$
B
$$1.44 \times {\sec ^{ - 1}}$$
C
$$0.72 \times {10^{ - 3}}{\sec ^{ - 1}}$$
D
$$2.88 \times {10^{ - 3}}{\sec ^{ - 1}}$$
Answer :
$$1.44 \times {10^{ - 3}}{\sec ^{ - 1}}$$